※This website contains information about products that may not be available in all countries. Nothing contained herein should be considered a solicitation, promotion or advertisement for any drug including the ones under development on this web site.Any information on the products contained herein is not intended to provide medical advice nor should be used as a substitute for the advice provided by your physician or other healthcare provider.
IN-HOUSE DEVELOPMENT
-

AVNeo (Aortic Valve Neo-cuspidization) Sizer System
– In-house development of an innovative device required for a cutting edge cardiac surgical procedure (AVNeo) developed in Japan.
– Product developed in-house (including license from a university)
– Distribution contracts signed with partners in 50 countries, currently expanding distribution markets (as of Feb 2023)
– See
owntissuevalve.org,
AVNeo.net ,
LinkedIn, and
facebook for more on this procedure.
-

3D back scanner® for scoliosis screening
–Space Vision, Inc. developed with Keio University.
–Launched by Nippon Zoki Pharmaceutical Co., Ltd.(
press release)
– LED light source and to capture an image of the patient’s back in 3D and convert it to a mirror image
–Supported by the JMA program, the development and commercialization of medical devices continues to progress (Japan Medical Association)
–Launched in Japan in February 2020 / globally unapproved
–Class I medical device (Japanese registration number 13B1X10274000004)
-
First Made in Japan Abdominal Aortic Aneurysm Stent Graft
– A stent graft entirely manufactured in Japan, highly competitive and features the concentration of Japanese technologies
– Aiming for unprecedented thin products under development
– Joint development with world renowned vascular surgeon, Professor Takao Ohki, and and key Japanese medical device manufacturers, MANI, Inc., and SB-Kawasumi Laboratories, Inc.
– In development / globally unapproved
-
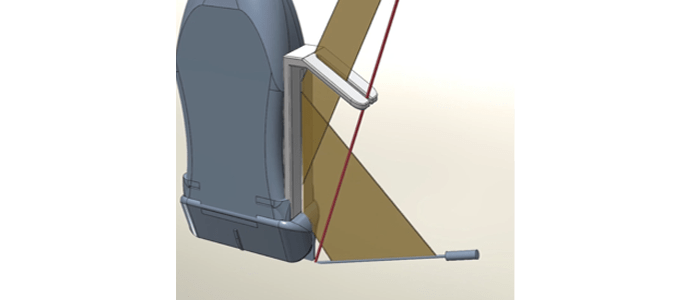
“True Puncture®”, a Needle Guide Device for Ultrasound-guided Puncture
Ultrasound guided puncture needle guide “True Puncture®”
– A device that enables practitioners to confirm appropriate line of sight, needle positioning and angle by placing a mirror on the puncture adapter
– An innovative guide system that enables a straight insertion of needles even for punctures made directly from the side
– Can be applied in cardiovascular, cardiac surgery, vascular surgery, and tumor-related surgeries (gastrointestinal surgery, respiratory surgery, breast surgery), orthopedic surgery, nephrology, etc.
– Class I medical device (Japanese registration number 8876083023)
-
-

AI-assisted automatic sperm sorting device
– In development by Dr. Kazuhiro Kawamura, International University of Health and Welfare, Dr. Masashi Ikeuchi, Tokyo Medical and Dental University, and Sanamedi
– Sanamedi will develop the device to order to aid in the embryologists’ sperm selection and improve workflow
– In development / globally unapproved
INVESTMENT
-
The World’s First Type Non-Invasive Pain Management Device with Unique Magnetic Field
– Joint development with P-Mind Co., Ltd. (
p-mind.co.jp)
– Theoretically expected to have an effect on all types of pain mediated by peripheral nerves with distinctive magnetic fields with multiple frequencies under a certain algorithm
– Starting with an indication for fibromyalgia, an intractable disease, treatment indications are expected to expand to include back pain, menstrual pain, joint pain, and pain after the treatment of external injuries
– In the mid- to long-term, seek to expand indications to include diseases of the central nervous system (dementia, mental illness, etc.)
– Class Ⅱ medical device
–Launched in Japan in 2022 / globally unapproved
-

Save Medical Corporation
Innovation in Digital Health and Digital Therapeutics
– Save Medical Corporation (
savemedical.jp) is developing an application for type 2 diabetes treatment
– Save Medical, established in May 2018 as a digital health focused subsidiary of Sanamedi
– In 2020, Save Medical conducted a clinical trial of an app for the treatment of type 2 diabetes for the first time in Japan.
-

“Hippocra×mynavi” Clinical Support Tool for Medical Doctor
Clinical mutual support tool “Hippocra×mynavi”
– Being provided by exMedio Inc. (
exmed.io)*Mynavi Corporation has acquired and made a subsidiary of exMedio Inc. in 2019
– A clinical consultation service that links specialists with non-specialists, a cross specialty introduction service
– Consultation requests can be made at any time, 24 hours per day, via smartphone / PC, and responses are received in approximately 30 minutes
– Service currently being provided in the dermatology and ophthalmology areas
– Enables the sharing of insights on treatment among physicians, discussions regarding the effects of a drug, searches for the latest papers, and viewing the latest medical news
-

Ultra-High-Speed and High-Sensitivity Cell/Bacteria Analysis Technology with Machine Learning
– In development by THINKCYTE, Inc.(
thinkcyte.com)*THINKCYTE completed JPY1.6 billion Series A Financing in 2020, and JPY2.8 billion Series B Financing
– Optical technology enabling high speed / high sensitivity cell and bacterial analysis
– Quantitative assessment of cells – abnormal cells, color, and N/C ratio (can be applied to cancer, cytology, and analysis of intestinal bacteria)
– Uses machine learning to detect abnormal cells
– Developing a support system in line with the test conditions of actual physicians and medical technologists from the “perspective of healthcare professionals”
– In development / globally unapproved
-

“MIRAI SPEAKER” , a universal speaker system for hard-of-hearing people
Barrier-Free Speaker “Mirai Speaker®”
– Developed and provided by SoundFun Inc. (
soundfun.co.jp)
– Speakers capable of clearly delivering information through sound to the ears of many with “curved sound” that generates sound from the entire curved diaphragm speaker with little attenuation or loss of clarity compared with conventional speakers.
– Is being adopted by major corporations including Japan Airlines, Nomura Securities, Resona Bank.
– Cooperative research: Chiba University, The University of Tokyo, Waseda University, Tokyo Metropolitan University
– Patent: 7 domestic patents, 8 overseas patents acquired, PCT world patents and Taiwan patents applied.
-

Photoacoustic Imaging (PAI) Technology
–In development by Luxonus Co. (
luxonus.jp )
–Fusion of two technologies, ultrasound imaging in the body and high resolution of optical imaging
–Ultra-high resolution 3D imaging of blood vessels and lymph vessels
–Aim to improve treatment results for diseases (vascular disorders, lymphedema, breast cancer, etc.) for which early diagnosis and disease diagnosis were difficult with existing imaging technologies
–The venture company was established on the basis of results from programs including the Japan Science and Technology Agency’s Impulsing Paradigm Change through Disruptive Technology Program (ImPACT)
– Acquired medical device manufacturing and marketing approval for photoacoustic imaging system LME-01 in 2022
-

Genome Service Platform
– In development by Genomelink, Inc.(
genomelink.io)
– Providing a reliable consumer platform to allow individuals access to their own genomic data
– Developing API software for business and individuals enabling the development of highly reliable integrated genomic services
– Establishing a platform for integrated genomic services worldwide
-

Robotics with High-Accuracy and Real-Time Biosignal Processing Algorithm
– Developed by MELTIN MMI K.K. (
meltin.jp)*MELTIN MMI completed JPY2.2 billion Series A and B Financing
– The fusion of biosignal processing using high precision / real-time algorithms and superior robotics / control technologies
– Realization of cyborg technologies through the Brain Machine Interface, linking brain to artificial body parts
– Launched in Japan in 2022 / globally unapproved
-

Innovative services in home nursing
– Developed and provided by Hospitality One K.K. (
hospitality-one.co.jp)
– A non-insured visiting nurse service with the mission to “create moments of peace” for many
– Currently developing a web-based service, “Discharge Support Navi”, for efficient collaboration between hospitals and visiting nurse stations
-

Innovative High-Resolution Ophthalmic Optical Coherence Tomography
Innovative, high-resolution ophthalmology OCT camera
– An OCT observation device that enables unprecedented resolution and depth through innovative light source technology
– In addition to diseases of the fundus such as glaucoma and age-related macular degeneration, it is expected to diagnose neurological diseases (such as Alzheimer’s disease) through the retina
– Not only will it enhance the accuracy of diagnoses, it will contribute to increasing patient QOL and reducing medical expenses by establishing the world’s first non-invasive / early diagnosis method
– Globally unapproved